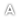Figure 1: Condition of the palm oil mill after incident
The accident has killed four workers and caused damage to a steriliser and building structures as shown in Figure 1 . The force of the explosion fractured the welded joint between the steriliser door and its support. This caused the door to flung about 11 meters away, while the 6 cages , which were processed at that time containing palm fruit were thrown out and scattered around the steriliser. During the event , all victim were working near the steriliser.
Preliminary investigation revealed the possibility of a failure at the steriliser door locking mechanism. Thus, this safety alert is specific to aspects related to door locking mechanism and recommendations shall be tailored to the type of machinery and manufacturer’s suggestions:
- Safety management system Important components such as training, working procedures, instructios dan work supervisions need to be emphasized.
- Inspection system
During operation, inspections on the following items shall be carried out such as:- Inspect alignment between door, locking ring and shell during the door opening/closing operation. Inspection should also consists of the lugs alignment(during the door was closed and locked) adhering to the minimum requirement recommend by the manufacturer’s
- Inspect physical condition of the steriliser such as shell wall inner surface; condition of lugs and wedges (located at locking ring, door and shell), integrity of the weld joints to detect any abnormality due to worn out or overloaded. Ensure that the tolerence of lugs and wedges should follow the specification and recommendation from the manufacturer;
- Inspect and maintain all door component (especially ajduster bolt, bearing and etc) and safety device (such as gasket, safety interlock, safety valve, steriliser door alignment and etc) installed to steriliser are functioning well. Inspection should also check the concentricity of its locking ring, door and shell, when the door was closed and locked. Dimensions and tolerence of lugs and wedges must conform according to the specification recommended by the manufacturer;
- Ensure any modification and repair work is approved by the Department and is done by competent person and firm; and
- All maintenance work must be monitered and ensured that it will not modify any original specifications on any parts of the steriliser.